Phase-0 Microdosing Studies Reported in the Literature
This section describes the properties of Phase-0 Microdosing in-vivo (humans and/or animals) studies reported in the literature. These studies expanded the methodology and/or applicability of Phase-0 approaches or were applied to actual novel drug development. There were 102 such studies as of November 2019. This is likely an underestimate since such studies are not mandated to be reported in the public domain. In addition, the list represents a likely underestimate since studies meeting the criteria of Phase-0 approaches are not always reported as such. The Phase-0 Microdosing studies list contains several studies with both microdose and therapeutic level exposures, normally not considered Phase-0 because of the therapeutic exposures, if they contributed to the validation of the approaches, study of their methodologies, or enabled the expansion of their applicability. Although thought to start in 2003 with the seminal publication by Lappin and Garner there are a couple of studies that meet the criteria and antedate that publication. Please contact us if you are aware of studies not included in the list.
Study Parameters
Objective: to identify publications of Phase-0 including Microdosing studies that were either used to validate and advance the methodology of these approaches or were applied in drug development.
Methodology: PubMed was searched for the terms ‘microdosing’, microdose’, ‘Phase-0’, ‘exploratory investigational new drug’, ‘exploratory IND’, ‘eIND’, and ‘exploratory clinical trials’ to identify index articles between 2000 and 2019. Additional studies were identified using the reference lists of the index articles. Reviews and concept manuscripts were excluded. Excluded were also publications that were only technical in nature or aimed at validating the tools used in Phase-0 approaches (e.g., liquid chromatography tandem mass spectrometers [LC-MS/MS]) rather than the approaches themselves.
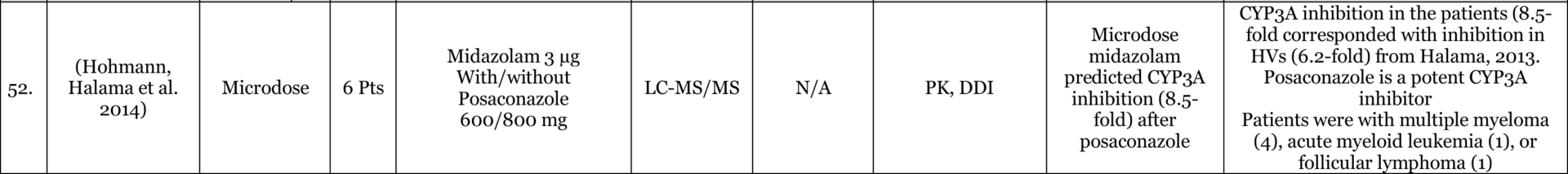
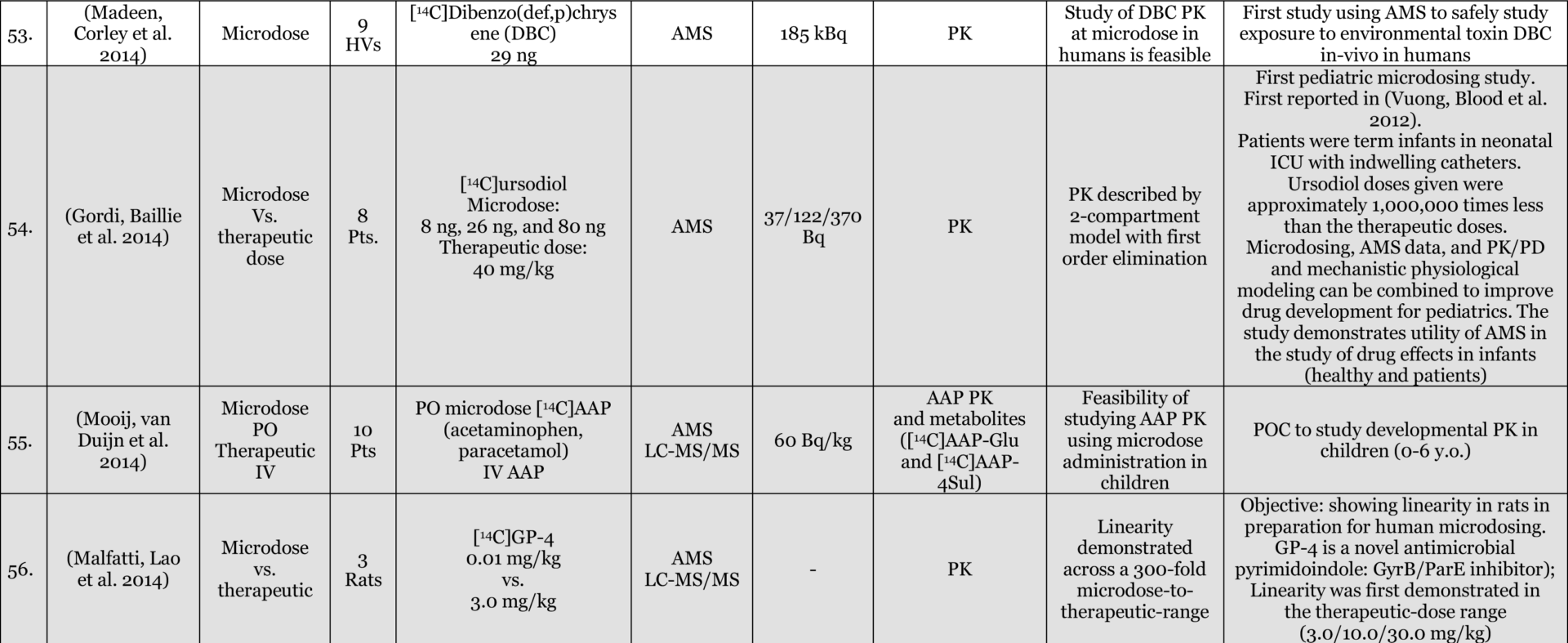
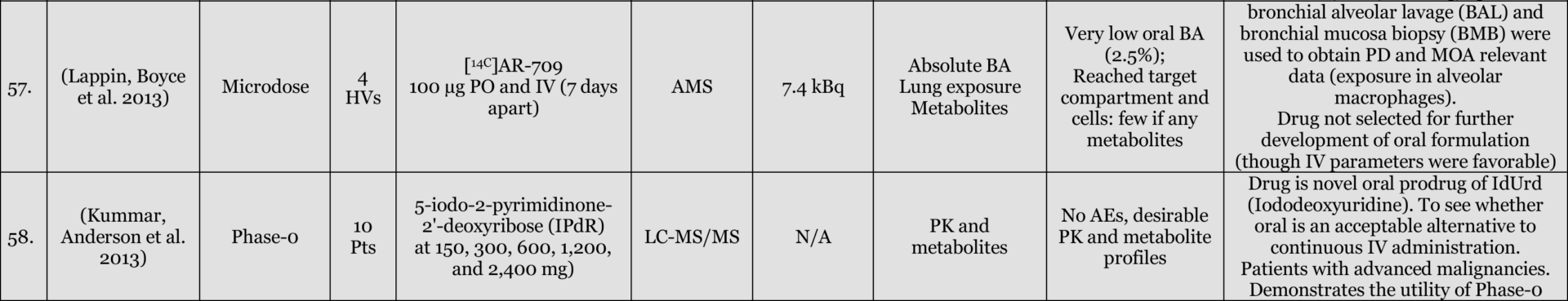
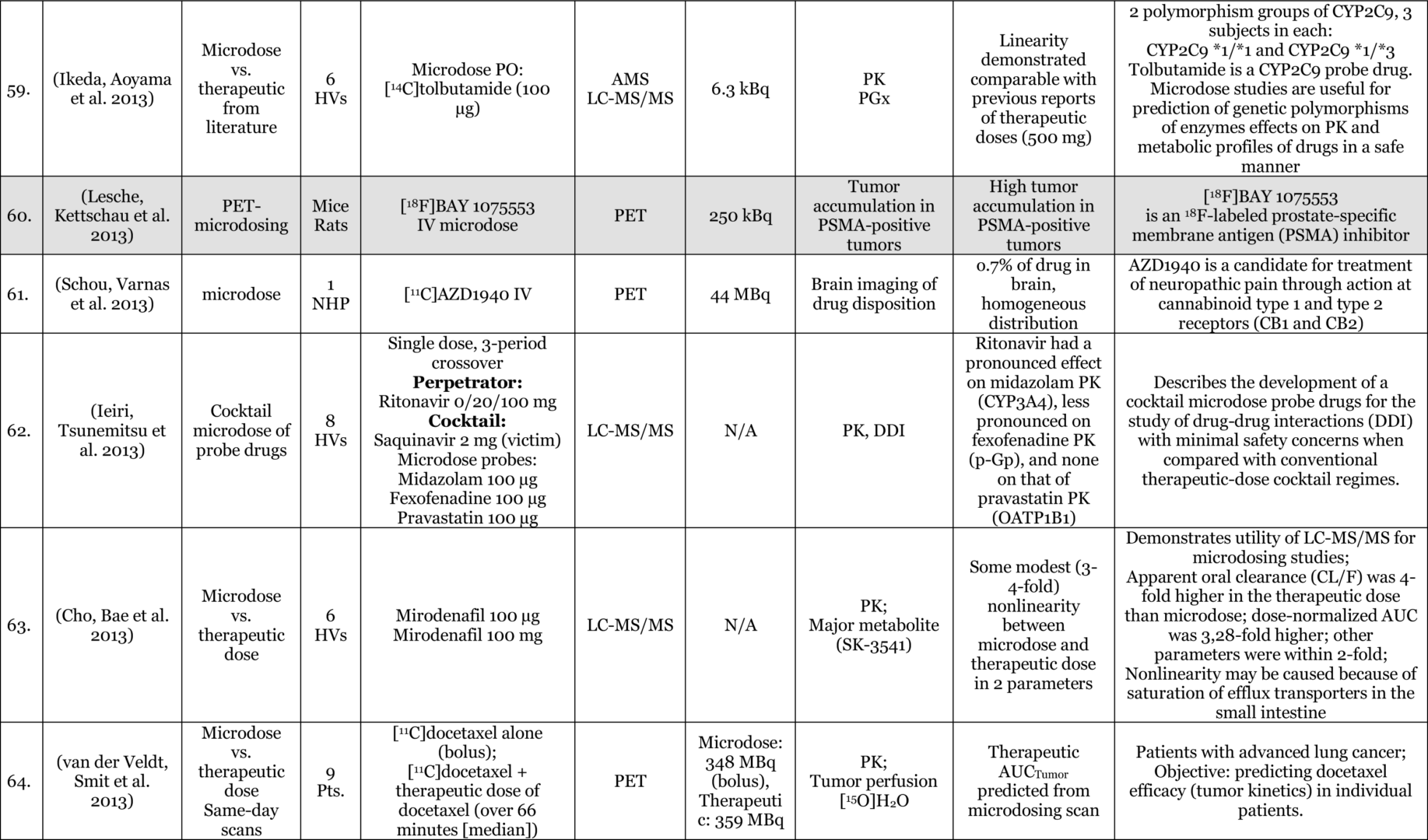
Results: Of 116 Phase-0 studies identified 107 (92%) are microdosing studies (consistent with ICH M3 [Table 3, p. 12] approaches 1 and 2) and 10 (9%) were non-microdosing Phase-0 studies (consistent with ICH M3 [Table 3, p. 12] approaches 3, 4, and 5) and one combined study (Kurdziel et al. 2019). The preference for microdosing studies probably reflects their greater appeal in most developmental scenarios due to the minimal regulatory package required to initiate them. Six (5%) studies were adaptive phase-0/phase-1 design, likely the most efficient approach in actual drug development. 54 (47%) reported on applications in actual drug development (either in novel drug development or development of approved drugs for novel populations [e.g., pediatric] or novel indications). 52 (45%) used LC-MS/MS (Liquid Chromatography Tandem Mass Spectrometry), 34 (29%) AMS (Accelerator Mass Spectrometry), 27 (23%) PET (Positron Emission Tomography), 4 (3%) NIRF (Near Infrared Fluorescence), 4 (3%) IHC (Immunohistochemistry), 2 (2%) SPECT (Single-photon emission computed tomography), and 1 (1%) ɣ-camera, with a few combinations. 64 (55%) of the studies included healthy volunteers, 37 (32%) included patients, 18 (16%) included animals with 4 (3%) of these being non-human primates, and a few combinations. The list excludes in-vitro studies except for 3 microdosing cell culture studies included because of their importance to the field and relevance to expansion of applications and methodological approaches.
Conclusions: the list provides a representation of the scope of research into, and applications of, Phase-0 including Microdosing approaches. The list is likely an underestimate of the number of studies used in drug development since reporting in the public domain is not required at this stage of development. In addition, there are Phase-0 studies that are published without the use of the keywords mentioned above (e.g., (Jonas, Landry et al. 2015, Jonas, Oudin et al. 2016)).
Phase-O / Microdosing Studies
Entries Highlighted with indicate Phase-o/Microdosing use in actual drug development
| 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|
| (Van Nuland, Rosing et al. 2020) | OL Microdose vs. Therapeutic dose | 10 pts. | Gemcitabine 100 μg Vs. Gemcitabine 1250 mg/m2 | LC-MS/MS | N/A | PK in plasma and intra-cellularly | Exposure was linear between microdose and therapeutic doses; However, Vd, elimination rate constant and intracellular PK were not linear | Gemcitabine and the metabolite 2',2'-difluorodeoxyuracil (dFdU) were studied |
| (Dunphy, Pressl et al. 2020) | PET microdosing | 30 pts | IV microdose (<25 μg) of [124]-PU-H71 | PET-CT | 201±12 MBq | Tumor detection; safety and feasibility | Drug detected various types of tumors and had favorable dosimetry; safe and feasible | This was a first-in-human study with [124]-PU-H71, an imaging agent for the imaging of epichaperome formations in cancer patients with breast, lymphoma, neuroblastoma, genitourinary, gynecologic, sarcoma, and pancreatic cancers. The study supported the continued clinical development of [124]-PU-H71 |
| (Zimmermann, Li et al. 2020) | 'diagnostic microdose' vs. Therapeutic dose | 6 pts | [14C]oxaliplatin in microdose Followed by FOLFOX | AMS | PK; DNA adducts in PBMCs | Linearity demonstrated between DNA adduct levels at microdose and therapeutic doses | The objective was to determine the value of [14C]oxaliplating 'diagnostic microdose; in determining response to FOLFOX therapy of colon cancer. The study indicates the potential utility of diagnostic microdosing | |
| (Mikus, Foerster et al. 2020) | Cassette microdosing | 6 HVs | Simultaneous microdoses of rivaroxaban, apixaban and edoxaban (100 μg in total) Together with either voriconazole, cobicistat or rifampicin (single dose) as perpetrators | LC-MS/MS | N/A | PK in plasma and urine | DDI was detected using the cassette microdosing approach | The objective was to study DDI after administration of cassette microdosing. The cassette microdosing administered drugs were all Factor Xa Inhibitors (FxaI). The study showed that cassette microdosing with this drug class was an effective way of studying DDI |